DI supported a global medical device manufacturer in SEA
• Client: A global medical device manufacturer
• Country: SEA
• Industry: Healthcare
Background:
A regional research division of a global medical equipment company specializing in ASEAN x Digital Health for 14 years with operations across Singapore and Thailand has struggled to produce tangible business outcomes. Despite significant R&D efforts driven by technology and product innovation, the team has not achieved any successful commercialization. This lack of results, combined with decentralized operations and unclear guidance from the head office, has led to inefficiencies and internal management concerns.
Facing increasing pressure from top management, the global headquarters seeks to reassess its R&D strategy and clarify its direction within the fiscal year. DI was engaged to conduct an objective evaluation of the R&D division’s capabilities and develop a clear strategic roadmap. The focus is on assessing its strengths in medical device development, manufacturing, and regulatory systems, as well as analyzing the necessity and strategic value of maintaining R&D bases in Singapore and Thailand to align with the company’s broader goals and mission.
Support Overview:
DI’s engagement centers on providing a comprehensive evaluation of the division’s R&D operations and formulating strategic recommendations. The key activities include:
Objective Capability Assessment: DI will analyze its strengths in medical device development, manufacturing, and regulatory compliance. This involves benchmarking its capabilities against industry standards and identifying core competencies that can be leveraged for commercialization.
Strategic R&D Base Analysis: DI will evaluate the necessity of maintaining R&D operations in Singapore and Thailand. This analysis will include cost-benefit assessments, alignment with market opportunities, and the strategic advantages of geographic positioning.
Competitor Benchmarking: DI will conduct a detailed study of comparable companies excelling in the ASEAN digital health and medical device sectors. Insights will help identify the best practices and potential areas for improvement in its R&D strategy.
Strategic Roadmap Development: Based on findings, DI will propose actionable recommendations to redefine its R&D focus. This includes prioritizing high-impact projects, addressing organizational inefficiencies, and outlining short- and mid-term objectives to align with management’s expectations.
DI’s work aims to provide the global headquarters with the clarity and direction needed to optimize its R&D operations and deliver measurable results. During the engagement, DI conducted an in-depth analysis of case studies from leading global companies in the same industry, along with extensive interviews with executives. The project was conducted covertly to ensure a fair and practical evaluation for the global headquarters.
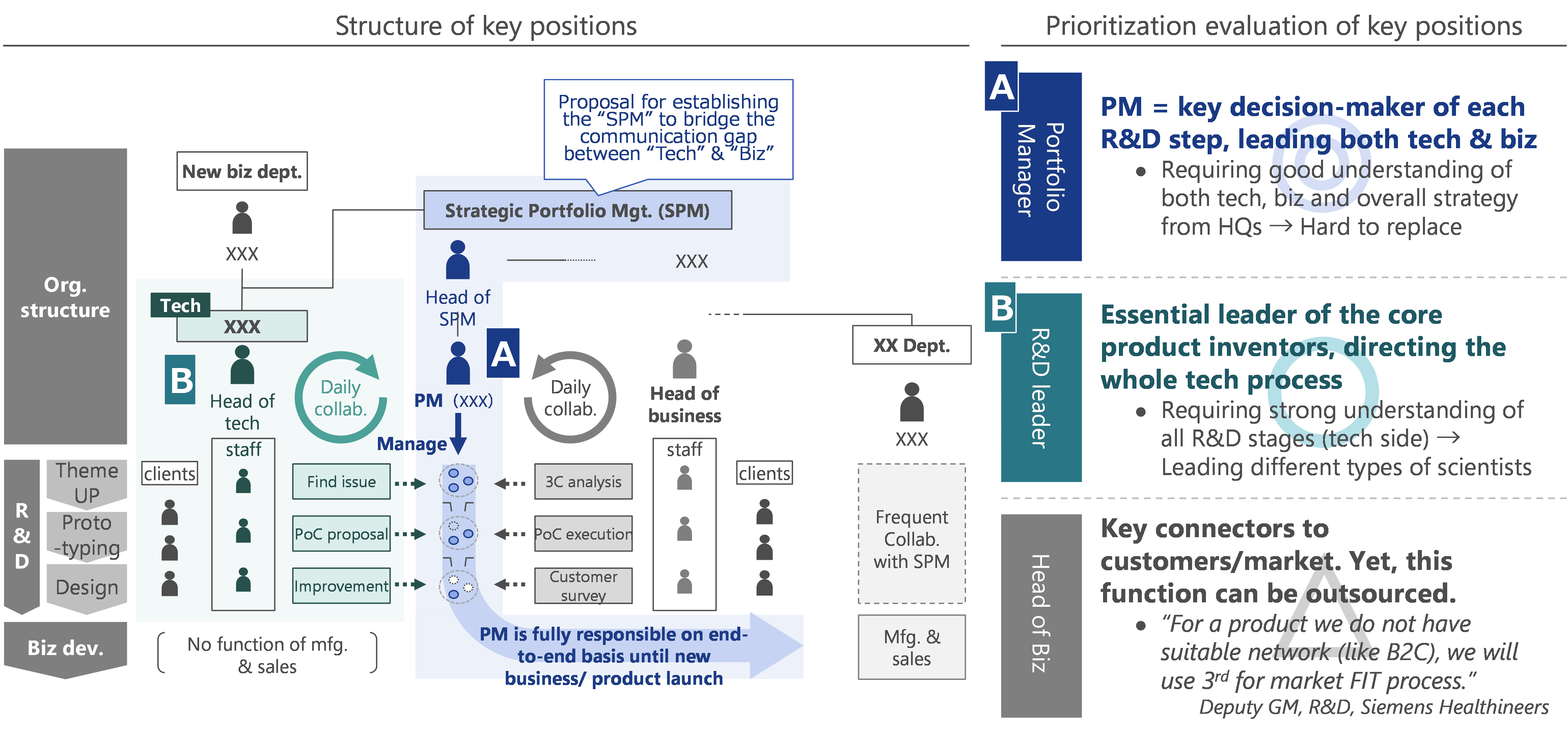
Image: excerpt from DI’s proposal for main themes of R&D organization structure model
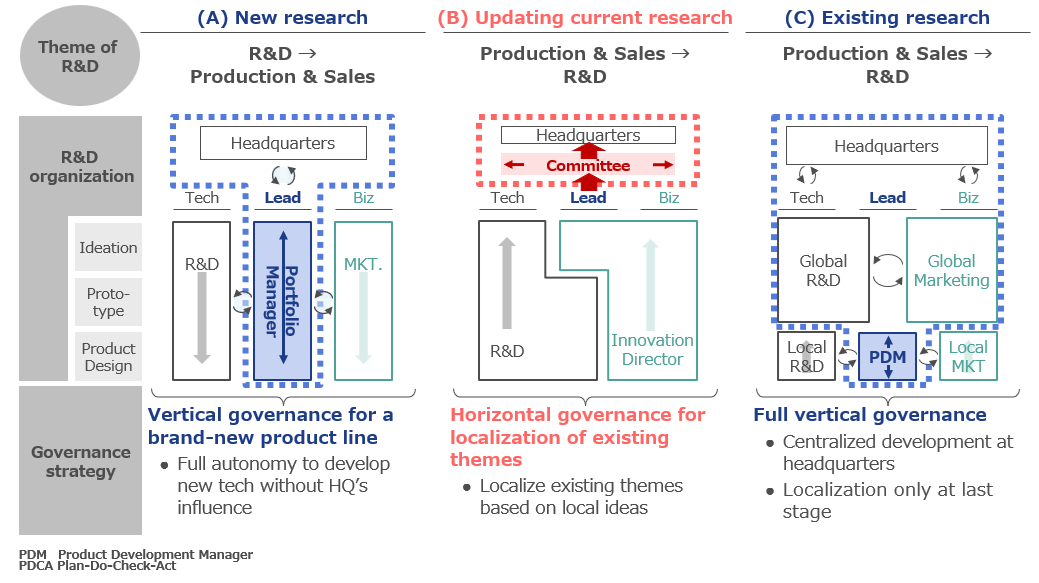
Image: complete support of DI from organization system design, key position prioritization until executive hunt strategy